超解像(SR)顕微鏡

Image at top left: SR microscopy (STORM) image of Cy5-Cy3 labeled tubulin from A431 cells.
Image courtesy of Prof. K. Lidke, University of New Mexico.
超解像(SR)顕微鏡は、蛍光を用いた光学顕微鏡の最近開発された手法を幅広く指すもので、レンズを使用した光学システムにおいて回折によって課せられる解像度の限界を巧妙に回避しています。
これらの超解像(SR)イメージング手法は、電子顕微鏡に匹敵する解像度を理論的に達成する可能性がありながら、はるかに安価で侵襲性が低いことから、基礎生物学研究の有力な研究ツールとして期待されています。
2014年のノーベル化学賞は、超解像蛍光顕微鏡の開発に対してエリック・ベツィグ、ステファン・W・ヘル、ウィリアム・E・モーナーに授与されます。
SR顕微鏡は、細胞生物学の理解を飛躍的に高める可能性があることから、2008年に「Nature Methods」によって「今年の方法」として選ばれました。
SR顕微鏡技術は、すでに10ナノメートル台の解像度を達成しており、細胞や巨大分子構造の画像を電子顕微鏡に近い解像度で生成しています。
SR顕微鏡技術は、全細胞や生きた細胞、さらには細胞構造にやさしい固定方法と互換性があります。さらに、非常に高い分子特異性を持つ多重ラベリングに使用でき、また、はるかに経済的で、実装も簡単である可能性があります。
これらの理由により、今後数年間でSR顕微鏡は分子レベルまで細胞プロセスの知識を飛躍的に向上させると広く期待されています。
レンズベースの光学顕微鏡による光の回折は、2つの物体を解像する力を制限します。この「回折限界」はエルンスト・アッベによって最初に認識され、それらを画像化するために使用される光の波長の約半分よりも近い2つの物体は、別々の物体として識別できないことを示しています。
実際には、利用可能な最高のレンズを使用すると、2つの解決可能なオブジェクト間の最小距離は、検出された光の波
長に応じて約200nmから350nmです。これが回折限界です。
SR顕微鏡は蛍光顕微鏡法に基づいており、サンプル内で特定の蛍光分子プローブを使用して、ある波長の光子を吸収し、続いて別の(通常はより長い)波長の光子を放出することができます。
これは、広視野で、またはサンプルをスキャンすることによって行うことができます。イメージング中の不要な波長の光による汚染を排除するために、適切なフィルターが使用されます。
蛍光顕微鏡のSR領域への拡張は、一部の蛍光分子に固有の重要な特性、つまり特定の波長の光を使用して、ある蛍
光状態から別の蛍光状態に「切り替える」または「活性化」する能力に依存します。
これらのプロセスは、それぞれ光スイッチングと光活性化として知られています。
また、サンプル中の蛍光プローブの吸収特性と発光特性を積極的に操作するために使用できます。
SR顕微鏡は、これらの特性を利用して、間隔の狭い2つの蛍光源から放出される光子を空間的に制限するか、または時間的に分離して別々にイメージングできるようにし、そうすることで回折限界を回避します。
新たなSR顕微鏡技術は、STED、RESLOFT、GSD、SSIM、fPALM、PALM、STORM、など、アルファベットの頭字語のスープに拍車をかけています。
これらの手法は、その詳細や実装方法が異なりますが、それぞれが蛍光プローブの特有の特性を利用しており、回折限界を超える分解能を達成することを示すために、総称して「超解像」と呼ばれています。
超解像顕微鏡技術の詳細と、Mad City Labs製品がSR顕微鏡アプリケーションでどのように使用できるかについては、以下の見出しをクリックしてください。見出しをもう一度クリックすると、セクションが折りたたまれます
The Nobel Prize in Chemistry for 2014 will be awarded to Eric Betzig, Stefan W. Hell, and William E. Moerner for the development of super-resolved fluorescence microscopy.
SR microscopy was chosen as the “Method of the Year” for 2008 by Nature Methods, based on its tremendous potential for increasing our understanding of cellular biology. SR microscopy methods have already achieved resolutions at the scale of 10s of nanometers, producing images of cellular and macromolecular structures with resolutions approaching those attained by electron microscopy.
SR microscopy methods are compatible with whole cells, or even live cells, as well as much more cell-structure-friendly fixation methods. Furthermore, they can be used for multiplex labeling with very high molecular specificity, and are also potentially much more economical and simpler to implement. For these and other reasons, it is widely expected that over the next several years, SR microscopy will lead to very
significant advances in our knowledge of cellular processes down to the molecular level.
The Diffraction Limit of Light The diffraction of light by lens-based optical microscopes limits their power to resolve two objects. This “diffraction limit” was first recognized by Ernst Abbe, and it dictates that two objects closer together than approximately half the wavelength of the light used to image them are not
discernible as separate objects. Practically speaking, with the best lenses available, the minimum distance between two resolvable objects is approximately 200nm to 350nm depending on the wavelength of light detected; this is the diffraction limit.
SR microscopy is based on fluorescence microscopy, which involves using specific fluorescent molecular probes within a sample that are capable of absorbing photons of one wavelength and then subsequently emitting photons of another (usually longer) wavelength. This can be done in either wide-field, or by scanning the sample.
Appropriate filters are used to eliminate contamination by unwanted wavelengths of light during imaging. The extension of fluorescent microscopy into the SR realm depends on critical properties intrinsic to some fluorescent molecules: the ability to “switch” or “activate” them from one fluorescent state to another using light of specific wavelengths. These processes are known as photo-switching and photo-activation, respectively; and they can be used to proactively manipulate the absorption and emission properties of the fluorescent probes in the sample.
SR microscopy exploits these properties to either spatially restrict, or temporally separate the photons emitted by two closely spaced fluorescent sources so that they can be imaged separately, and in doing so circumvents the diffraction limit.
SR Microscopy Techniques
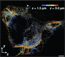
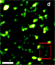
Image at left from York AG, Ghitani A, Vaziri A, Davidson MW, Shroff H. Confined activation and subdiffractive localization enables whole-cell PALM with genetically expressed probes. Nature Methods, Vol. 8, No. 4. (13 April 2011), pp. 327-333. Figure 5(b).
PALM is Photo-activated Localization Microscopy, fPALM is fluorescence Photo-activated Localization Microscopy, and STORM is Stochastic Optical Reconstruction Microscopy. PALM, fPALM, and STORM are “localization-based” techniques that take advantage of the photo-activation and photo-switching properties of fluorescent probes in a manner that differs from STED and SSIM. If two point sources of light are emitting photons of the same wavelength simultaneously, the equation of Ernst Abbe given above still governs the resolving power of any microscope. However, if these point sources can be made to emit their photons individually, at different times so that they can be separately collected, the diffraction limit can be circumvented. In localization-based SR imaging, the fluorophore labels are switched on and off stochastically in sparse subsets while imaging these subsets en masse using an EMCCD camera. This is done in the wide-field, rather than by scanning the lasers. Super-resolution is achieved by localizing each fluorescent emitter by computing the center of the point spread function (PSF) of each one as measured by its photon density count on an array of pixels on the EMCCD camera. The final image is built from a stack of many such images, each representing only a small fraction of the total number of the individual fluorescent emitters in the entire field. Localization-based SR microscopy techniques have already achieved resolutions in the realm of 10-20nm in the x-y plane and may have the potential to achieve even higher resolutions.
STED is Stimulated Emission Depletion Microscopy. STED microscopy is one of the methods that spatially restricts fluorophore excitation and emission. In this technique, the excitation volume is reduced through the use of two lasers. The first laser excites the fluorophores, while the second laser turns the fluorophores off. By surrounding the first laser spot by a second one with a donut-shaped intensity profile, and adjusting the intensities these two lasers appropriately, the excitation volume can be reduced below the diffraction limit. This technique is fundamentally a scanning-based method, and the excitation and depletion lasers are moved rapidly through the sample while the emitted photons are collected, frame-by-frame, by an electron-multiplying charge-coupled device (EMCCD) camera as this process proceeds. These frames are then assembled into a complete image of the entire field. GSD (ground state depletion) and RESOLFT (reversibly saturable optical fluorescence transitions) are fundamentally similar to STED. STED has produced images of biological samples with lateral resolutions in the 60nm range.
SSIM is Saturated Structured Illumination Microscopy. SSIM is a technique that illuminates the sample with wide-field patterned light, and the excitation pattern interacts with the sample’s spatial information resulting in Moiré fringe patterns being produced. The information contained within these fringes can be computationally extracted, and an image of the sample can be calculated using this information that goes beyond the diffraction limit. SSIM has attained resolutions in the 50-100nm range.
New Developments in SR Microscopy at Mad City Labs

Image at left of Mad City Labs Nano-LPS Series XYZ nanopositioning stage
Stay up to date with new developments by following Mad City Labs’ Super-Resolution Microscopy blog.
.jpg)
Nano-Cyte® is a 3D image stabilization system for microscopy. With Nano-Cyte® you no longer need to be concerned with temperature gradients, sample drift, and microscope drift. Unprecedented stability in the nanometer regime allows the extension of single molecule techniques into the realm of cell biology.
Recommended Systems for SR Microscopy

Image at left of Mad City Labs Nano-F Series objective nanopositioner
| Product | Description | Axes | Applications |
| Nano-Cyte™LC | 3D image-based stability for live cell imaging | 3 | Super-resolution (SR) Microscopy, Live Cell Imaging, Single Molecule Microscopy |
| Nano-View® Series | Fully integrated nanopositioning and micropositioning systems for use with inverted optical microscopes, combined with a high resolution, 2-axis or 3-axis nanopositioner | 2 or 3 | STORM, PALM, confocal and fluorescence imaging, single molecule microscopy and spectroscopy, particle tracking, optical trapping, optical tweezers, super resolution (SR) microscopy |
| Nano-View®/M Series | Fully integrated nanopositioning and micropositioning systems for use with inverted optical microscopes that offer long range manual micropositioning in two axes, combined with a 2-axis or 3-axis nanopositioner | 2 or 3 | STORM, PALM, confocal and fluorescence imaging, single molecule microscopy and spectroscopy, particle tracking, optical trapping, optical tweezers, super resolution (SR) microscopy |
| Nano-LPS Series | Low profile system specifically designed for optical microscopy with a arge aperture (83mm) | 3 | STORM, PALM, confocal and fluorescence imaging, alignment, super resolution (SR) microscopy |
| Nano-LP Series | Low profile with 100µm, 200 µm or 300 µm in X, Y and Z. | 3 | single molecule microscopy and spectroscopy, fluorescence imaging, super resolution (SR) microscopyy |
| Nano-BioS Series | Ultra low profile system designed to be easily integrated into existing inverted microscopes, AFM’s and other instrumentation | 2 | STORM, PALM, confocal and fluorescence imaging, nanolithography, super resolution (SR) microscopy |
| Nano-Bio Series | Low profile, with large aperture for use with inverted optical microscopes, available in 50 µm, 100 µm, and 200 µm ranges of motion | 2 | optical microscopy, AFM scanning, super resolution (SR) microscopy |
| Nano-T Series | Economical system with XY motion up to 200 µm and Z-axis motion up to 50 µm | 2 or 3 | fluorescence imaging, super resolution (SR) microscopy, AFM scanning |
| Nano-Z Series | 100 µm or 200 µm range of motion, large center aperture, and low profile | 1 (Z) | super resolution (SR) microscopy, optical microscopy |
| Nano-F Series | Objective lens focusing elements with 100 µm or 200 µm of travel | 1 | STORM, PALM, confocal and fluorescence imaging, super resolution (SR) microscopy |
| C-Focus™ System | Focus stabilization system that eliminates microscope focus drift over long time periods | 1 | microscope focus correction, high speed confocal imaging |
| Nano-F3D | Objective lens nanopositioner with 3-axis (XYZ) motion up to 100 µm per axis | 3 | 4Pi microscopy, custom scanning microscopy applications, optical inspection |
Examples of Mad City Labs Systems in SR Microscopy Research
| Image at left from Q. Li, S. S. H. Wu, and K. C. Chou. Sub-diffraction-limit Two-photon Fluorescence Microscopy for GFP-tagged Cell Imaging. Biophys. J . 97, 3224 (2009). Figure 2(d) |
Below are selected publications from customers:
- Yan Q, Schwartz SL, Maji S, Huang F, Szent-Gyorgyi C, Lidke DS, Lidke KA, Bruchez MP. Localization Microscopy using Noncovalent Fluorogen Activation by Genetically Encoded Fluorogen-Activating Proteins. ChemPhysChem. 2013.
École Polytechnique Fédérale de Lausanne (EPFL), Laboratory of Nanoscale Biology (LBEN)
- P. Annibale, M. Scarselli, M. Greco and A. Radenovic. Identification of the factors affecting co-localization precision for quantitative multicolor localization microscopy. Optical Nanoscopy 2012, 1:9
Harvard University, Chemistry, Zhuang Lab
- Joshua C. Vaughan, Shu Jia, and Xiaowei Zhuang Ultrabright photoactivatable fluorophores created by reductive caging. Nat Meth. 2012 published online Oct 28.
- Sang-Hee Shim, Chenglong Xia, Guisheng Zhong, Hazen P. Babcock, Joshua C. Vaughan, Bo Huang, Xun Wang, Cheng Xu, Guo-Qiang Bi, and Xiaowei Zhuang Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. PNAS 2012 : 1201882109v1-6.
- Jones SA, Shim SH, He J, Zhuang X. Fast, three-dimensional super-resolution imaging of live cells. Nat Methods. 2011 Jun;8(6):499-505.
- A. Dani, B. Huang, J. Bergan, C. Dulac, X. Zhuang, Super-resolution imaging of chemical synapses in the brain, Neuron 68, 843-856 (2010).
- B. Huang, S.A. Jones, B. Brandenburg, X. Zhuang, Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution, Nature Methods 5, 1047-1052 (2008).
- M. Wu, B. Huang, M. Graham, A. Raimondi, J.E. Heuser, X. Zhuang, P. De Camilli, Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system, Nature Cell Biology 12, 902-908 (2010).
Max Planck Institute for Biophysical Chemistry, Department of Nanobiophotonics
- Wurm, C. A., K. Kolmakov, F. Göttfert, H. Ta, M. Bossi, H. Schill, S. Berning, S. Jakobs, G. Donnert, V. N. Belov, S. W. Hell. Novel red fluorophores with superior performance in STED microscopy. Optical Nanoscopy 2012, 1:7.
- York AG, Ghitani A, Vaziri A, Davidson MW, Shroff H. Confined activation and subdiffractive localization enables whole-cell PALM with genetically expressed probes. Nature Methods, Vol. 8, No. 4. (13 April 2011), pp. 327-333.
University of British Columbia, Chemistry, Keng C. Chou
- Q. Li, S. S. H. Wu, and K. C. Chou. Sub-diffraction-limit Two-photon Fluorescence Microscopy for GFP-tagged Cell Imaging. Biophys. J . 97, 3224 (2009).
University of New Mexico, Physics and Astronomy, Lidke
- K van den Dries, SL Schwartz, J Byars, MB Meddens, M Bolomini-Vittori, DS Lidke, CG Figdor, KA Lidke, A Cambi, Dual color super-resolution microscopy reveals nanoscale organization of mechanosensory podosomes. Molecular Biology of the Cell.2013 Jul; 24(13):2112-23.
- F. Huang, S.L. Schwartz, J.M. Byars, and K.A. Lidke, Simultaneous multiple-emitter fitting for single molecule super-resolution imaging. Biomedical Optics Express, Vol. 2, Issue 5, pp.1377-1393 (2011)
- Smith, C.S., N. Joseph, B. Rieger, and K.A. Lidke, Fast, single-molecule localization that achieves theoretically minimum uncertainty. Nature Methods, 2010. 7(5): p. 373-U52.
- Huang, Fang; Schwartz, Samantha L.; Byars, Jason M.; and Lidke, Keith A. Simultaneous multiple-emitter fitting for single molecule super-resolution imaging. Biomed Opt Express. 2011 May 1; 2(5): 1377–1393.
Yale University, Department of Biological & Biomedical Sciences, Bewersdorf Lab
- S. Liu, E. B. Kromann, W. D. Krueger, J. Bewersdorf, K. A. Lidke, Three dimensional single molecule localization using a phase retrieved pupil function. Optics Express, 21:29462-29487 (2013)
